Introduction: Why FDA Direct Portal Expertise Is Non-Negotiable
In today’s highly regulated U.S. market, US FDA compliance is not optional—it is foundational. Drug manufacturers, private label distributors, outsourcing facilities, cosmetic brands, and logistics stakeholders must comply with strict federal registration, listing, and reporting obligations to legally operate in the United States.
The FDA Direct Portals, particularly CDER Direct and Cosmetics Direct, are the official electronic gateways for submitting regulatory data to the U.S. Food and Drug Administration and its drug division, Center for Drug Evaluation and Research.
However, navigating these portals—especially Structured Product Labeling (SPL), Drug Establishment Registration, NDC Labeler Code requests, and MoCRA cosmetic registrations—requires deep regulatory and technical expertise. This is where XPRO America US FDA Consultants become a strategic partner rather than just a service provider.
Understanding FDA CDER Direct: The Backbone of Drug Compliance
4
CDER Direct is the FDA’s centralized submission platform for drug-related regulatory obligations. It is used by:
- Drug manufacturers
- Private label distributors
- Generic drug facilities
- Outsourcing facilities (503B)
- Wholesale drug distributors
- Third-party logistics (3PL) providers
Key CDER Direct Submission Sections
1. Establishment Registration & Drug Listing
Every facility engaged in manufacturing, repacking, relabeling, or distributing drugs in U.S. commercial distribution must register annually with FDA and list all applicable drug products.
XPRO America manages:
- FDA Establishment Registration (initial & annual renewal)
- Drug Product Listing via SPL
- Updates for formulation, labeling, or manufacturing changes
2. NDC Labeler Code Requests & Reservations
The National Drug Code (NDC) is mandatory for drugs marketed in the U.S. CDER Direct allows:
- New NDC Labeler Code applications
- NDC product and package code reservations
Errors here can delay product launch. XPRO America ensures accurate SPL mapping, correct dosage forms, and compliant labeling hierarchies.
3. Outsourcing Facility & Product Reporting (503B)
Outsourcing facilities must submit:
- Facility registration
- Semiannual drug product reports
XPRO America handles:
- Facility classification
- Product SPL creation
- Timely FDA submissions
4. DSCSA Annual Reporting
Under the Drug Supply Chain Security Act (DSCSA), manufacturers and repackagers must submit annual reports to FDA confirming compliance with traceability and supply chain integrity requirements.
5. Generic Drug Self-Identification
Generic drug facilities must self-identify annually, linking products, ANDA references, and manufacturing roles. XPRO America ensures precise data alignment to avoid FDA discrepancies.
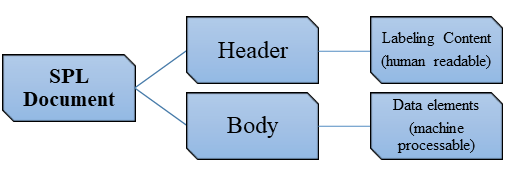
Structured Product Labeling (SPL): The Technical Core

4
SPL is not a PDF or Word document—it is an XML-based data standard mandated by FDA. A single tagging error can result in rejection.
XPRO America provides:
- SPL authoring & validation
- XML schema compliance
- FDA ESG/CDER Direct upload support
- Lifecycle management for SPL updates
This technical-regulatory bridge is where most companies fail without expert consultants.
Cosmetics Direct & MoCRA: A New Era of Cosmetic Regulation


4
MoCRA Legal Background
On December 29, 2022, the U.S. President signed the Consolidated Appropriations Act, 2023 (Pub. L. 117-328) into law. This legislation included the Modernization of Cosmetics Regulation Act of 2022 (MoCRA), amending the Federal Food, Drug, and Cosmetic Act (FD&C Act) by adding Section 607.
What MoCRA Mandates
Under MoCRA, cosmetic companies must now complete:
- Cosmetic Facility Registration
- Manufacturers and processors (domestic & foreign)
- Mandatory updates for ownership or location changes
- Cosmetic Product Listing
- Each cosmetic product marketed in the U.S.
- Ingredient disclosures, brand details, and manufacturing sites
All submissions must be made via Cosmetics Direct, the FDA’s official portal.
Why Hire XPRO America for Cosmetics Direct Compliance
XPRO America supports:
- MoCRA facility registration
- Cosmetic product listing
- Amendments & annual updates
- U.S. Agent coordination for foreign manufacturers
They ensure:
- Correct ingredient nomenclature
- Alignment with FDA cosmetic definitions
- Error-free FDA portal submissions
Coverage Across the Entire FDA-Regulated Ecosystem


4
- Drug manufacturers (innovator & generic)
- Private label distributors
- Outsourcing facilities (503B)
- Wholesale drug distributors
- Third-party logistics providers (3PLs)
- Cosmetic brands & contract manufacturers
Their consultants understand how FDA evaluates commercial distribution status, facility roles, and product classifications.
Why XPRO America Is a Strategic FDA Partner (Not Just a Consultant)
1. Direct Portal Expertise
Hands-on experience with CDER Direct and Cosmetics Direct, not theoretical compliance.
2. Zero-Error Submission Philosophy
FDA rejections cost time, money, and market access. XPRO America focuses on first-time-right submissions.
3. End-to-End Lifecycle Management
From initial registration to annual renewals, SPL updates, and regulatory changes.
4. Ideal for U.S. & Non-U.S. Companies
Foreign manufacturers benefit from XPRO America’s U.S. Agent support and FDA communication handling.
Business Impact: Compliance That Enables Revenue
FDA compliance is not a checkbox—it is a revenue enabler. Without valid registrations, listings, and MoCRA filings:
- Products can be detained or refused at U.S. ports
- Amazon, Walmart, and U.S. distributors may reject onboarding
- Investors and partners lose confidence
Hiring XPRO America ensures your regulatory foundation supports scalable U.S. commercialization.
Conclusion: Secure FDA Compliance with XPRO America
Whether you are submitting Drug Establishment Registration, NDC Labeler Codes, SPL drug listings, DSCSA reports, or MoCRA cosmetic registrations, the FDA Direct portals demand precision, experience, and accountability.
XPRO America US FDA Consultants provide exactly that—trusted expertise for CDER Direct and Cosmetics Direct, ensuring your drugs and cosmetics remain compliant, market-ready, and commercially viable in the United States.
US FDA Consultants, XPRO America, CDER Direct, FDA Direct Portal, Drug Establishment Registration, Drug Listing SPL, Structured Product Labeling, NDC Labeler Code, NDC Reservation, Outsourcing Facility Registration, DSCSA Annual Reporting, Generic Drug Self Identification, Cosmetics Direct, MoCRA Compliance, Cosmetic Facility Registration, Cosmetic Product Listing, US FDA CDER, FD&C Act Section 607, FDA Drug Compliance Consultant, FDA Cosmetic Registration
Leave a Reply