
US FDA registered drug manufacturers play a critical role in supplying safe, effective, and compliant pharmaceutical products to the United States market. Any company involved in the manufacturing, processing, packing, or labeling of drugs intended for the US must comply with strict regulatory requirements set by the United States Food and Drug Administration. Understanding what FDA registration means, who needs it, and how compliance is maintained is essential for both domestic and international pharmaceutical manufacturers.
What Does FDA Registration Mean for Drug Manufacturers?
FDA drug establishment registration is a mandatory legal requirement under US law. It applies to companies that manufacture finished pharmaceuticals, active pharmaceutical ingredients (APIs), repack drugs, or relabel products for the US market. Registration does not mean product approval; instead, it confirms that the manufacturing facility is known to the FDA and subject to regulatory oversight.
Once registered, a drug manufacturer is listed in the FDA’s database and assigned a unique establishment identifier. This enables the FDA to conduct inspections, monitor compliance, and communicate regulatory updates efficiently.
Who Must Register as an FDA Drug Manufacturer?
FDA registration applies to both US-based and foreign drug manufacturers. If a company produces drugs that are imported into the United States, registration is mandatory regardless of the manufacturing location. This includes manufacturers in India, Europe, China, and other global pharmaceutical hubs.
Foreign drug manufacturers must also appoint a US FDA U.S. Agent. The U.S. Agent acts as the official point of contact between the FDA and the overseas facility, ensuring timely communication during inspections, compliance queries, or emergency situations.
FDA Registration and GMP Compliance
Registration alone is not enough. FDA registered drug manufacturers must fully comply with Current Good Manufacturing Practices (cGMP) as outlined in 21 CFR Parts 210 and 211. These regulations govern every aspect of pharmaceutical manufacturing, including facility design, equipment validation, quality systems, documentation, and staff training.
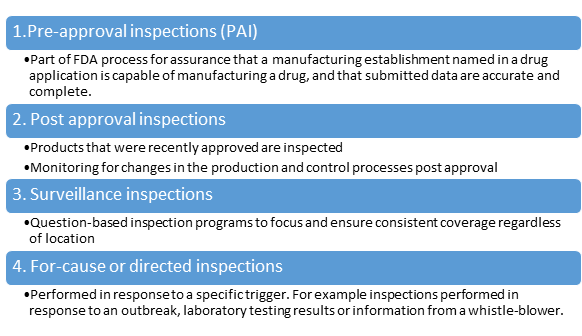
FDA inspections are conducted on a risk-based schedule. During inspections, investigators assess whether the facility consistently produces drugs that meet quality and safety standards. Observations may result in Form 483s, warning letters, or import alerts if serious deficiencies are identified.

Benefits of Being an US FDA Registered Drug Manufacturer
Becoming FDA registered offers significant commercial and regulatory advantages. It allows manufacturers to legally supply drugs to the US market, enhances global credibility, and builds trust with distributors and partners. FDA registration is often viewed as a benchmark of quality, even in non-US markets.
For contract manufacturers and API producers, FDA registration can open doors to partnerships with multinational pharmaceutical companies that require FDA-compliant supply chains.
Common Challenges for Drug Manufacturers
Despite its importance, FDA registration and compliance can be complex. Common challenges include incomplete documentation, inadequate quality systems, poor data integrity practices, and lack of inspection readiness. For foreign manufacturers, navigating US regulatory expectations and communication protocols can be particularly demanding.
These challenges highlight the importance of working with experienced regulatory professionals who understand FDA requirements in depth.
How a US FDA Consultancy Can Help
Partnering with a specialized US FDA Consultancy can significantly reduce compliance risks and delays. XPRO America, a trusted US FDA Consultancy, supports drug manufacturers with FDA drug establishment registration, U.S. Agent services, GMP readiness, and inspection support. Their regulatory expertise helps manufacturers align operations with FDA expectations and maintain long-term compliance.
If you are planning to register your drug manufacturing facility or need ongoing FDA regulatory assistance, guidance from experienced consultants can make the process smoother and more reliable. For professional support related to FDA registered drug manufacturers, you can connect with the regulatory team at support@xproamerica.com to discuss your specific requirements.
Staying Compliant After Registration
FDA compliance is an ongoing responsibility. Registered drug manufacturers must renew their registration annually, update listings promptly, and maintain inspection readiness at all times. Continuous improvement of quality systems and proactive regulatory monitoring are key to long-term success in the US pharmaceutical market.
For companies aiming to build a strong and sustainable presence in the United States, FDA registration is not just a regulatory obligation—it is a strategic investment in quality, credibility, and global growth.
Leave a Reply